- Procedure from order to delivery
- Material Transfer Agreement
- List of required documents
- Fee
- Where to send required documents
- Payment
- Delivery
- Terms of use
- Contacts
1. Procedure from order to delivery
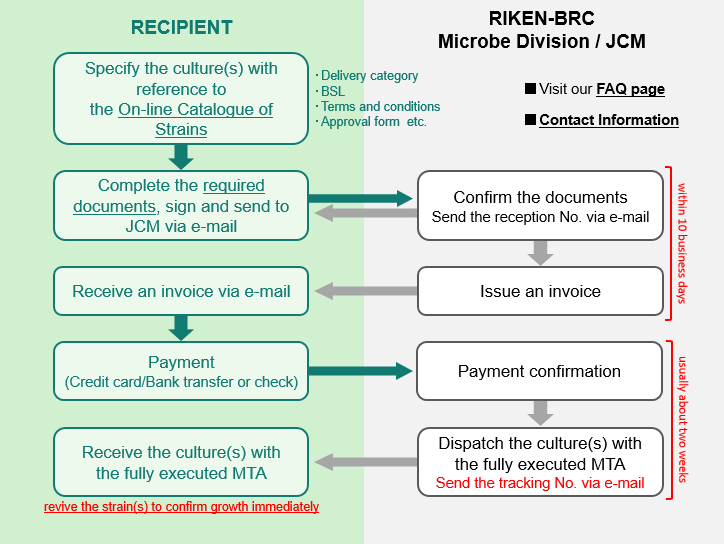
- Specify both scientific name(s) and JCM accession number(s) on the ORDER FORM with reference to the on-line catalogue of strains.
- APPROVAL FORM (if requested)
Check the on-line catalogue of strains to see if an APPROVAL FORM is requested. If requested, you must have the form signed by the DEPOSITOR and sent back to you before your order can be processed. - Documents
Complete the required documents (see the list that follows), sign and send to the address shown below (see No. 5). - ORDER FORM (Form M-10)
- MATERIAL TRANSFER AGREEMENT (2 original copies)
- Category I MTA (Form M-11) or
- Category II MTA (Form M-11C)
- APPROVAL FORM (Form M-12. Only if requested)
- ACCEPTANCE OF RESPONSIBILITY FOR POTENTIALLY PATHOGENIC MATERIALS (Form M-13): This form is necessary when the order is for a strain to be handled in Biosafety Level 2 facilities (including those listed as “provisional”). Please check the on-line catalogue to determine whether or not the strain of your interest should be handled at Biosafety Level 2.
- ACCEPTANCE OF RESPONSIBILITY FOR LIVING A MODIFIED ORGANISM (Form M-13G): This form is necessary when the order is for a “living modified organism (LMO)” in order to comply with the Cartagena Protocol on Biosafety to the Convention on Biological Diversity. Please check the on-line catalogue to learn whether or not the strain of your interest is considered an LMO. We will provide you with the required information for the LMO.
- Invoice from RIKEN-BRC
- We will review your order and send you an invoice and the payment instruction by e-mail as a PDF attachment.
- Payment
- Dispatch of the culture(s) by JCM when the MTA (for distribution) is concluded and payment is received. An automated message will be sent to you when the culture(s) is sent out.
2. Material Transfer Agreement
- When a culture strain is to be supplied, a Material Transfer Agreement (MTA) is concluded between the RECIPIENT and RIKEN BioResource Research Center, to promote the utilization of biological resources and to clarify rights and obligations for use of the strain.
- The research topic must be indicated in the MTA. A new MTA is required if there is a significant change in the intended use of the strain(s).
- Please use the appropriate category of the Material Transfer Agreement (MTA);
- Category I MTA: For not-for-profit academic research by not-for-profit institutions.
- Category II MTA: Applicable for the following:
- For research to be conducted by for-profit organization.
- For collaborative research by not-for-profit organization with for-profit organization.
- For research by not-for-profit organization outsourced and sponsored by for-profit organization.
- For for-profit research by not-for-profit organization, including R&D with the aim of patent acquisition.
- In addition to Category I or II MTA, certain resources require prior written permission of the Depositor.
- Some strains listed in the catalogue have specific terms and conditions that were set up to protect the DEPOSITOR’s property at the time it was deposited. If the requested strain is one of these, the same terms and conditions are set forth in the MTA, and the RECIPIENT must abide by them.
- When ordering a strain that requires an “APPROVAL FORM (Form M-12)” from the DEPOSITOR, the RECIPIENT must contact the DEPOSITOR beforehand. The “APPROVAL FORM” must be completed and sent to the DEPOSITOR to obtain approval before your order is placed.
- The same MTA can be used again so long as the research topic and intended use are the same and all parties (person responsible for the transaction, collaborative researcher(s), and head of relevant institution) are in agreement. Indicate the MTA registration number on the ORDER FORM when placing an order based on a formerly concluded MTA.
- Papers and reports concerning research using the supplied resources should indicate that the resources were supplied by RIKEN BRC. You are also asked to send one copy of the paper or report to RIKEN BRC so that it can be listed on the BRC website and included in the resource data.
- Strains listed in the on-line catalogue may be unavailable or their supply requirements according to maintenance conditions, regulations or other circumstances.
- In addition to the terms and conditions specified by the depositors on the MTA, use of a biological resource could be regulated due to the implementation of the Nagoya Protocol, or domestic laws in the country of origin or the provider country. Therefore, users are encouraged to check regulations and obtain any permissions necessary to conduct research and development. Any relevant information that has been provided by depositors or related authorities is disclosed in the online catalogue.
| Forms | Number of copies | Form required | Sample | ||
|---|---|---|---|---|---|
| Form M-10 |
ORDER FORM [Excel] (latest version : 2026-01-05) ORDER FORM [PDF] (payment by bank transfer) (latest version : 2026-01-05) ORDER FORM [PDF] (payment by credit card) (latest version : 2026-01-05) (You may choose bank transfer or credit card for your payment. If you choose the payment by credit card, we will send an e-mail with the subject, “Online payment from RIKEN BioResource Research Center” to your registered e-mail address from this page.) |
1 copy | for each order | How to prepare Form M-10 | |
| Form M-11 |
MATERIAL TRANSFER AGREEMENT (FOR DISTRIBUTION) (about category) | Category I MTA (latest version : 2023-08-17) | 2 original copies | for each order | How to prepare Form M-11 |
| Category II MTA (latest version : 2023-08-17) | How to prepare Form M-11C | ||||
| Form M-12 |
APPROVAL FORM (latest version : 2018-04-01) | 1 copy | if requested | How to prepare Form M-12 | |
| Form M-13 |
ACCEPTANCE OF RESPONSIBILITY FOR POTENTIALLY PATHOGENIC MATERIALS (latest version : 2022-08-05) | 1 copy | for a strain to be handled at Biosafety Level 2 | How to prepare Form M-13 | |
| Form M-13G |
ACCEPTANCE OF RESPONSIBILITY FOR LIVING MODIFIED ORGANISM (latest version : 2024-10-10) | 1 copy per strain | for a “Living Modified Organism” | – | |
- The Forms listed above can be downloaded as Adobe® PDF files. Please complete the forms on-screen with Adobe® Reader®, sign them, and then send them to the address shown below (see No. 5) by post or electric transmission as a PDF file.
Please confirm the delivery category with reference to the on-line catalogue of strains. Shipping costs will be charged to overseas users. If the governmental postal service (“post”) is selected as carrier, the fee for shipping will be included in the invoice. If the forwarder is selected, the shipping company should be arranged for by the Recipient, and the fee for shipping should be handled cash on delivery (COD). If required, an import permit/license should be obtained and provided when the order is placed.
| Delivery category | Unit | Carrier * | Fee (Japanese yen) | |||
|---|---|---|---|---|---|---|
| Post | Forwarder | For use in research for not-for-profit academic purpose | For use in research for-profit-research purpose | |||
| A | Freeze-dried or L-dried culture † | ampoule or tube | ○ | ○ | ¥5,500 | ¥11,000 |
| F | Frozen culture ‡ | tube or vial | ☓ | ○ | ¥7,480 | ¥14,960 |
| B | Actively growing culture of a microbial strain that cannot be preserved as a dried culture (solid or liquid medium, ca. 5-20 mL) | tube or plate or vial | ○ | ◎ | ¥11,000 | ¥22,000 |
| C | Actively growing culture on request (solid or liquid medium, ca. 5-20 mL) | tube or plate or vial | ○ | ◎ | ¥19,030 | ¥38,060 |
| D | Microbial genomic DNA (1 µg) see <https://dna.brc.riken.jp/en/jcmdnaen> | ¥19,910 | ¥39,820 | |||
* ◎ recommended, ○ acceptable, ☓ unavailable
† Including a frozen-thawed culture (in a plastic tube) of some fungi.
‡ Only available for the recipient who can arrange the shipment by the courier that satisfies the following conditions.
The courier should:
· accept microbes.
· have a Japan branch.
· provide dry ice with the Dry Ice Shipper or liquid nitrogen with the Liquid Nitrogen Shipper for the transport.
· provide a service to replenish dry ice at the customs (upon arrival to the customs) such as World Courier and Cryoport.
Please note that the dry ice should be refilled upon arrival to customs, NOT AFTER passed the customs.
| Special discount system for large-volume orders | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
For single orders of 10 or more cultures, the total charge will be reduced according to the number of cultures as shown in the table below.
|
||||||||||
5. Where to send required documents
| Microbe Division / Japan Collection of Microorganisms RIKEN BioResource Research Center 3-1-1 Koyadai, Tsukuba, Ibaraki 305-0074, Japan E-mail: inquiry.jcm |
Billing will be handled by the BioResource Research Center Planning Office, RIKEN. Please remit payment in accordance with the instructions in the bill.
<< Please note! >>
Payments must be prepaid.
The method of payment cannot be changed after we start processing to issue the invoice.
RIKEN is a non-profit organization and therefore requires all applicants to cover all bank charges (including exchange, commission and handling fees) when sending payments for resources.
Please note the followings when you remit the distribution fee for BRC’s bioresources from your bank.
- If the remitter’s name differs from the addressee on the invoice sent by BRC, we may not accept the remittance. If you wish to pay under a name different from the BRC invoice, please consult with BRC reception in advance.
- If the remitter’s name differs from the addressee on the BRC invoice, BRC will confirm with the billing recipient (i.e., the ordering party). This process may delay the shipment of BRC’s bioresources.
- If payment is made to BRC from an institution or individual with a name different from the addressee on the BRC invoice without prior notification, a refund may not be possible.
The culture(s) is shipped when the MTA (for distribution) is concluded and payment is received. In general, a microbial strain is sent as a freeze-dried or L-dried culture in a glass ampoule. Please refer to the instructions provided with the ampoule or here to revive the culture. In case of a strain that cannot be preserved as a dried culture, the strain is sent as an active culture in a glass tube, a frozen-culture in a plastic tube or a frozen-thawed culture in a plastic tube. For the latter, please refer to the instructions provided with the tube or here to revive the culture. If there are any problems in a delivered culture, please inform JCM referring to No. 9.
Please read the terms below. You are deemed to accept them when placing your order.
(1) Possible cases for compensation and report deadline
| Cases for compensation | Report deadline (after the shipping date) |
|---|---|
| ➀ Resource undelivered, missing or damaged, frozen resource already thawed on arrival, etc. | within 14 days |
| ➁ Unable to recover and culture with the designated medium and conditions, contamination, etc. | within 30 days |
| ➂ Unable to confirm the properties of the bioresource described in the catalogue, etc. | within 3 months |
If you experience such a case as stated above, please send your written notification or email to the Japan Collection of Microorganisms (inquiry.jcm![]() riken.jp) by the deadline shown. We will take necessary action immediately.
riken.jp) by the deadline shown. We will take necessary action immediately.
(2) Conditions and limitations for compensation
In a case of ①, ② or ③reported before the designated deadline, we will replace the strain you order with the same or an alternative strain one time only free of charge or a refund. The refund will not exceed the distribution fee. Overseas users will be charged the shipping cost for replacement. No compensation will be made in a case of failure or damage caused by the user.
(3) Cancellation fee
Upon receipt of an order from a user, we will start preparing the requested resource by the required processes including microbe cultivation. These preparations are very costly. Should a user cancel the order as a matter of user’s convenience, the user will be charged for the costs we have spent on preparation up to the cancellation notification (up to 100% of the distribution fee). Notification of cancellation after BRC has sent out the resource will result in 100% of the distribution fee plus shipping cost being charged to the user.
(4) Accident during shipment
Our Center is not liable for any accident that occurs during shipping. Please select a carrier and make necessary arrangements on your own responsibility.
For overseas shipment, a user is responsible for customs clearance including preparation of necessary documents.
(5) Information release on resources with quality defect
Whenever we note a quality defect in our resource, we will announce it through our website. We will also individually contact users who received such a resource in the previous 3 years.
To inquire about microbial strains:
| Microbe Division / Japan Collection of Microorganisms RIKEN BioResource Research Center 3-1-1 Koyadai, Tsukuba, Ibaraki 305-0074, Japan E-mail: inquiry.jcm |
To inquire about payment:
| BioResource Research Center Planning Office RIKEN 3-1-1 Koyadai, Tsukuba, Ibaraki 305-0074, Japan E-mail: brc-front |




