- Procedure for Deposition of a microorganism
- Documents required
- Import-prohibited microorganism by the Plant Protection Act
- Shipment
- Address to which documents and microbial cultures should be sent
- Notification of JCM accession number
- Certificate of deposition and availability of a microorganism
- Contact
1. Procedure for Deposition of a microorganism(s)
JCM accepts bacteria, archaea, yeasts, filamentous fungi and related microorganisms that can be handled in Biosafety Level 1 or 2 facilities (corresponding to Risk Group 1 or 2; JCM does not accept microorganisms classified in Risk Group 3 or higher). To protect the DEPOSITOR’s rights and to promote the utilization of biological resources in the field of microbiological research and development, a MATERIAL TRANSFER AGREEMENT (MTA) should be executed between the DEPOSITOR and RIKEN BioResource Research Center.
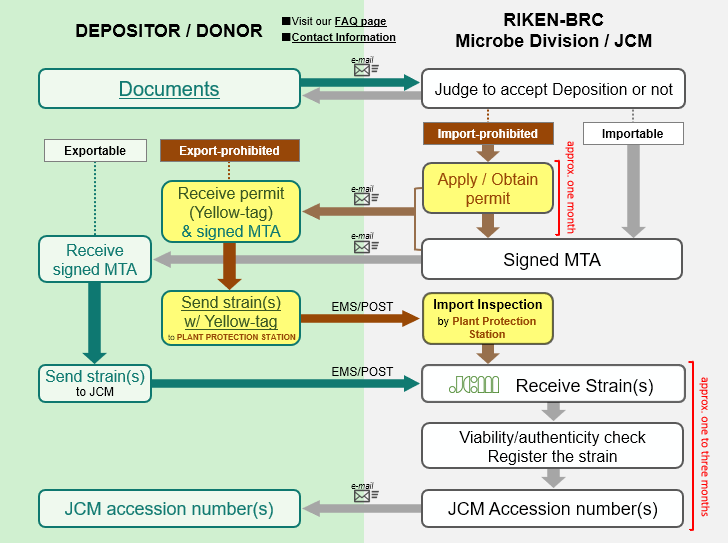
An outline of the procedure for depositing a microorganism in JCM is as follows:
1) The DEPOSITOR prepares a “DEPOSITION DATA SHEET” (for BACTERIA AND ARCHAEA or YEASTS or FILAMENTOUS FUNGI) per strain and “MATERIAL TRANSFER AGREEMENT (FOR DEPOSITION)”. If the strain is a “living modified organism” or “genetically modified organism”, additional documents are also required as described below. Regarding each document, JCM accepts only the latest version of the form whose mandatory items are filled in without omission.
2) The DEPOSITOR sends the above-mentioned documents by e-mail to <inquiry.jcm![]() riken.jp> as scanned images prior to sending the culture. The documents are looked over to determine whether JCM can accept the organism with or without permission from the Japanese authorities in the light of Japanese laws, e.g. the Plant Protection Act, etc. The DEPOSITOR may be asked to make corrections to the documents at this step.
riken.jp> as scanned images prior to sending the culture. The documents are looked over to determine whether JCM can accept the organism with or without permission from the Japanese authorities in the light of Japanese laws, e.g. the Plant Protection Act, etc. The DEPOSITOR may be asked to make corrections to the documents at this step.
3) After JCM informs the DEPOSITOR of a decision on acceptance, the DEPOSITOR is free to choose between 1) allowing JCM to work with the scanned MATERIAL TRANSFER AGREEMENT FOR DEPOSITION / DONATION or 2) sending the original two copies of MATERIAL TRANSFER AGREEMENT FOR DEPOSITION / DONATION by postal service to the address shown below.
4) If the depositing microorganisms are import-prohibited, the procedures added to the procedure for deposition of importable microorganisms (1. applying for an import permit, 2. obtaining the import permit) add around 1 to 3 months to the process.
5) After the MTA between the DEPOSITOR and RIKEN BRC is concluded and one copy of the MTA is returned to the DEPOSITOR, the DEPOSITOR sends the culture with a cover letter indicating the DEPOSITOR’s name and affiliation, the scientfic name and strain designation of the organism, and the MTA number. JCM does not accept a culture without advance notice and conclusion of an MTA in any case.
6) A JCM accession number is assigned to the microorganism and the DEPOSITOR is informed of the number after authenticity of the culture is confirmed by checking viability and purity and by sequencing an appropriate gene like a ribosomal RNA gene.
| Forms (Please download the latest version) | |||||
|---|---|---|---|---|---|
| No. | Documents | Forms | No. | Form required | Fill out sample |
| i. | DEPOSITION DATA SHEET | for BACTERIA AND ARCHAEA (Latest version:2021-02-08) | M-8-P | 1 copy /strain | – |
| for FILAMENTOUS FUNGI AND YEASTS (Latest version:2021-02-08) | M-8-F | 1 copy /strain | – | ||
| ii. | MATERIAL TRANSFER AGREEMENT | for Donation (Latest version:2021-04-08) | M-2E | How to prepare Form M-2E | |
| for Deposition (Latest version:2021-04-08) | M-9 | How to prepare Form M-9 | |||
| iii. | “LIVING MODIFIED ORGANISM” or “GENETICALLY MODIFIED ORGANISM” | LEGALLY REQUIRED INFORMATION FOR LIVING MODIFIED ORGANISM | M-8-G | 1 copy /strain | – |
| DATA SHEET OF LIVING MODIFIED ORGANISM | M-8-GD | 1 copy /strain | – | ||
| Microbe Division / Japan Collection of Microorganisms RIKEN BioResource Research Center 3-1-1 Koyadai, Tsukuba, Ibaraki 305-0074, Japan Fax: +81 29 836 9561 E-mail: inquiry.jcm |
6. Notification of JCM accession number
The JCM accession number will be assigned to the microorganism and the DEPOSITOR will be informed of the number after the culture has been checked and preserved.
7. Certificate of deposition and availability of a microorganism
In accordance with the policy of the International Journal of Systematic and Evolutionary Microbiology (IJSEM), authors who wish to propose a new species, a new subspecies or a new combination in IJSEM or to apply for citation of a new name in the Validation List of IJSEM are required to prove deposition of the type strain in culture collections and its availability to the public without restriction. In response to this policy and upon request by a depositor, JCM issues a certificate of deposition and availability to the DEPOSITOR or one of authors of a manuscript describing a new taxon. The certificate can be issued after confirmation of the viability and purity of the deposited culture and also confirmation of its authenticity by the 16S rRNA gene analysis. Please contact us to apply for the certificate.
JCM issues the certificate only for deposited strains that can be distributed without restriction for academic research purpose. If terms and conditions in the MTA (for Deposition) restrict distribution or require acquisition of a written consent using the Approval Form (Form M-12) for academic research purpose, JCM does not issue the certificate in response to the policy of IJSEM.
| Microbe Division / Japan Collection of Microorganisms RIKEN BioResource Research Center 3-1-1 Koyadai, Tsukuba, Ibaraki 305-0074, Japan Fax: +81 29 836 9561 E-mail: inquiry.jcm |






